Key Findings
CAD III confirms IVL safety with low rates of major peri-procedural clinical and angiographic complications, setting a new bar for safety in complex coronary lesions.
-
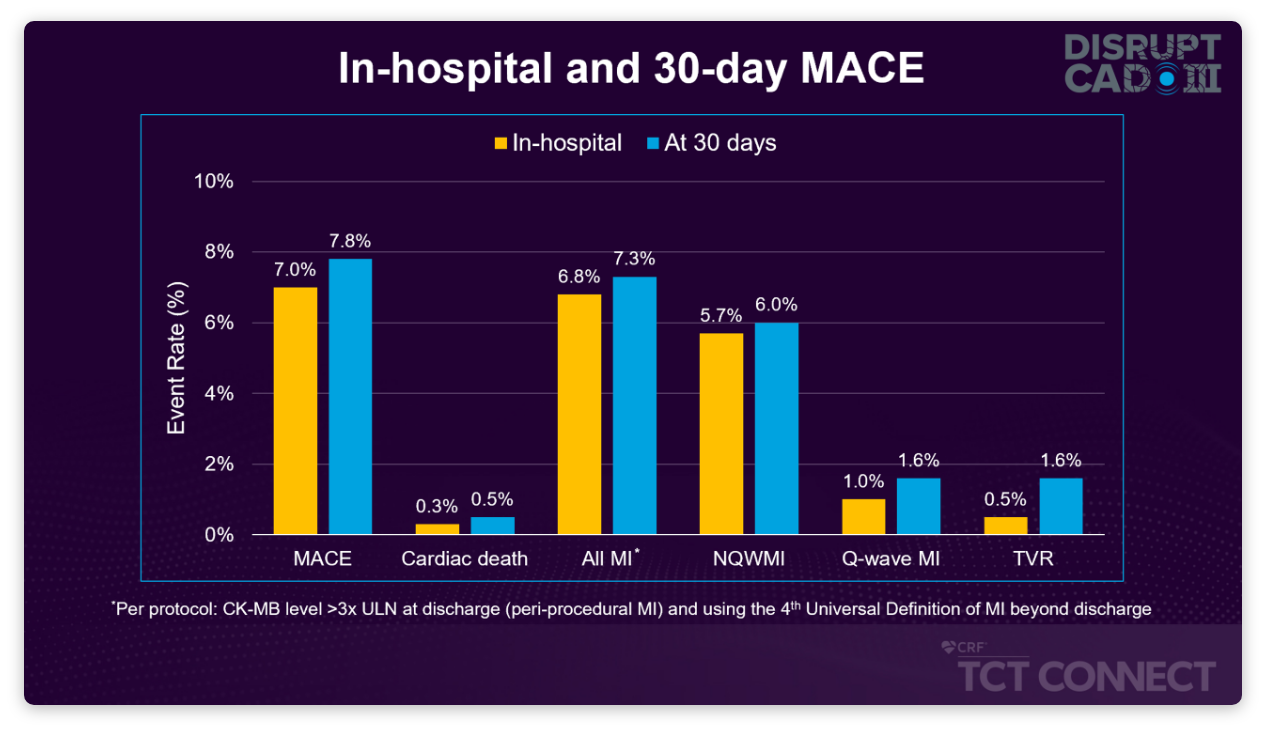
92.2% of patients were free from MACE at 30 days, a composite of CD (0.5%), MI (7.3%), or TVR (1.6%)
- Primary MACE driver was in-hospital non-Q-wave MI (5.7%)
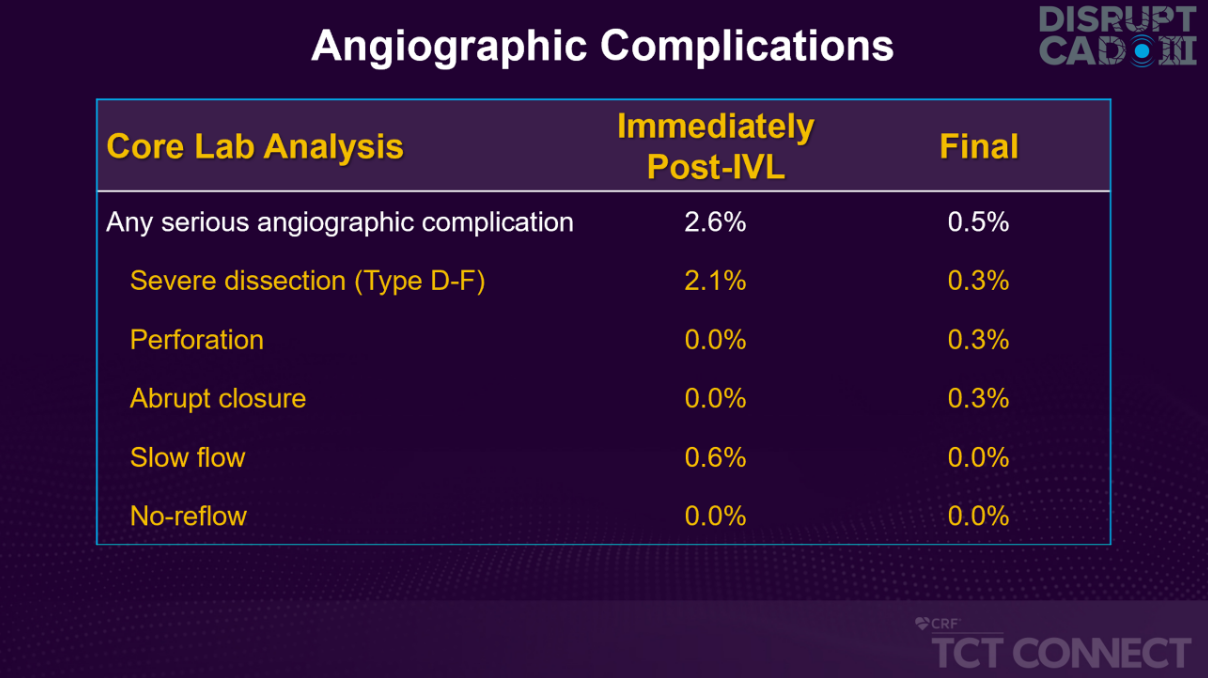
- Low risk of perforation (0.3%), major dissection (0.3%), abrupt closure (0.3%), and slow flow/no reflow (0.0%) at the end of procedure
CAD III showcases IVL effectiveness with large lumen gains that facilitate stent delivery and optimize stent expansion.
- 92.4% procedural success rate, defined as successful stent delivery (99%) residual stenosis <50% (100%), & without in-hospital MACE (93%)
- Successful IVL crossing & therapy delivery in 98% of lesions, correlating to 99% stent delivery
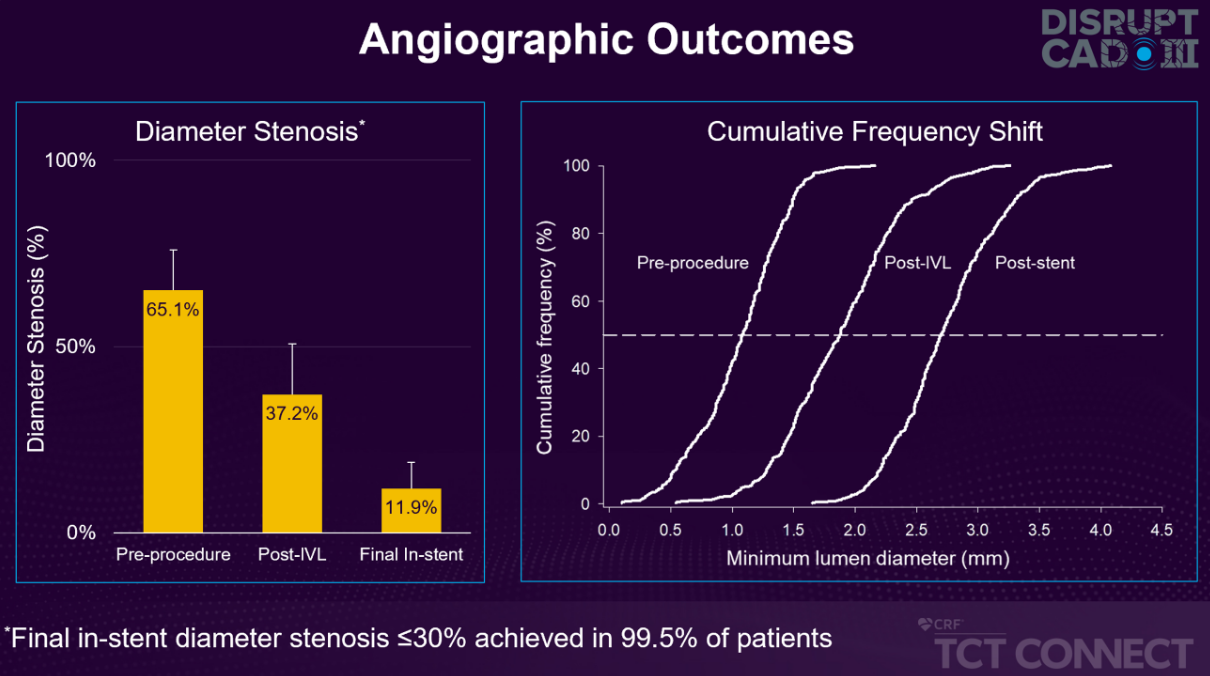
- 1.7mm acute gain and 11.9% final in-stent residual stenosis
CAD III demonstrated Coronary IVL’s ease of use and quick learning curve to achieve consistently predictable outcomes.
- Despite >80% of operators having no prior experience with IVL, MACE, procedural success and device crossing success were similar between first roll-in first case and pivotal cohort

CAD III By The Numbers
DOWNLOAD CAD III HIGHLIGHTS47 Sites
Delivery
Dissections
DISRUPT CAD III OCT Sub-Study Provides New Insights on IVL’s Unique MOA that Facilitates Optimal Stent Delivery, Expansion and Apposition


Top 10 Data Points to Know Download CAD III INFOGRAPHIC
DISRUPT CAD III Design & Patient Demographics

Objective: Prospective, multicenter, single-arm global IDE to evaluate the safety and effectiveness of coronary IVL
Primary Safety Endpoint: Freedom from MACE at 30 days
- Cardiac death, Myocardial infarction, or Target vessel revascularization
Primary Effectiveness Endpoint: Procedural success
- Successful stent delivery with residual stenosis <50% and without in-hospital MACE
Secondary Performance Endpoints: Clinical and Angiographic Success
Baseline Characteristics:
71 yo
40% DM
3.0mm RVD
65% DS
48mm Ca++ Length
25mm Lesion Length
57% LAD, 13% LCX, 29% RCA, 1% LM
292 Ca++Arc @ Max Ca++ Site
.96mm Thick Ca++ @ Max Ca++ Site
Large Circumferential Lumen Gains to Facilitate Stent Delivery, Apposition & Expansion
Low Rates of Complications, Similar to DISRUPT CAD I & II
Low Rates of MACE, Mostly Driven by In-Hospital NQWMI